- New data from in vitro, in vivo, and IND enabling studies supports LP-284’s development for MCL, an aggressive form of B-cell non-Hodgkin's lymphoma (NHL) with immediate patient needs.
- Lantern is anticipating filing the IND with the FDA in early 2023 and initiating a first-in-human Phase 1 trial for LP-284 in NHLs, including MCL and double hit lymphoma (DHL), by mid 2023.
- In the US and Europe, MCL and DHL are diagnosed in approximately 9,000 patients each year and have an estimated annual market potential of $1.2 billion USD.
Lantern Pharma Inc. (NASDAQ: LTRN), a clinical stage biopharmaceutical company using its proprietary RADR® artificial intelligence ("A.I.") and machine learning (“M.L.”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, today announced it presented new positive preclinical data for its drug candidate LP-284 for Mantle Cell Lymphoma (MCL) at the American Society of Hematology Annual meeting.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221215005355/en/
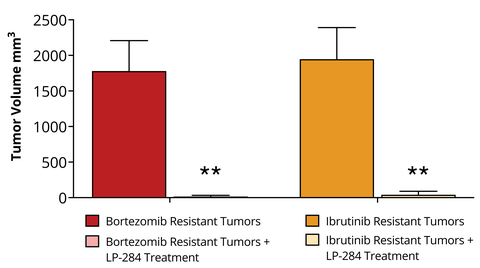
Figure 1. In mice implanted with MCL CDX tumors that had been treated and then grown resistant to Bortezomib or Ibrutinib, subsequent LP-284 treatment of 4 mg/kg (i.v.) resulted in near complete tumor regression in the SOC resistant MCL CDX tumors (** p < 0.01). (Photo: Business Wire)
The ASH poster highlights new results for LP-284 from preclinical studies for MCL and initial results from investigational new drug (IND) enabling studies. LP-284 treatment was demonstrated to have significantly greater tumor growth inhibition (TGI) in mice implanted with MCL cell derived xenograft (CDX) tumors, when compared to treatment with the standard-of-care (SOC) agents Ibrutinib or Bortezomib (see Table 1).
Table 1.
Agent (Dose; Administration) |
LP-284 (4 mg/kg; i.v.) |
LP-284 (2 mg/kg; i.v.) |
Bortezomib (1 mg/kg; i.p.) |
Ibrutinib (50 mg/kg; p.o.) |
TGI (%) |
113% |
63% |
22% |
8% |
Table Legend: Tumor growth inhibition (TGI); Intravenous (i.v.); Intraperitoneal (i.p.); Oral (p.o.) |
||||
Additionally, in mouse MCL CDX tumors that had been treated and then grown resistant to either Ibrutinib or Bortezomib, subsequent LP-284 treatment of 4 mg/kg (i.v.) resulted in near complete tumor regression in the SOC resistant MCL CDX tumors (see Figure 1). These new promising results are critically important from a clinical perspective as nearly all MCL patients eventually relapse from Ibrutinib and Bortezomib treatment.
New in vitro data was also presented that identified a potential mechanism of LP-284’s anti-tumor activity in MCL. LP-284 treatment of MCL cell lines significantly down-regulated key cancer promoting genes and pathways, including the onco-fusion gene CCND1 and genes in the MYC pathway. Combined, these new in vitro and in vivo preclinical data for LP-284 strongly support its anti-tumor activity in MCL over current SOC agents and its continued advancement towards a Phase 1 clinical trial.
“This compelling pre-clinical efficacy and tumor response data, in both new lymphomas and those that had become resistant to standard of care agents, is an exciting advancement for LP-284 in hematological cancers and positions Lantern to advance our discussions with biopharma companies for partnering and collaborative development opportunities,” stated Panna Sharma, Lantern’s CEO and President. “Even with the vanguard of new CAR T-cell therapy approaches in B-cell cancers, there is a critical need for improved options for B-cell cancer patients that don’t qualify for, have access to, or can’t afford CAR T-cell therapy,” continued Sharma.
The ASH poster also shows initial results from the large animal non-GLP toxicology portion of the IND enabling studies for LP-284, where the no observed adverse effect level (NOAEL) of LP-284 was determined to be 0.3 mg/kg/dose. Establishment of the NOAEL will facilitate the completion of IND enabling studies, which Lantern is anticipating in Q1 of 2023, followed by an anticipated launch of a first in human Phase 1 clinical trial later in 2023.
A full version of the poster presentation can be found on Lantern’s website.
About LP-284:
LP-284 is a novel small molecule and DNA damaging agent being developed by Lantern for the treatment of several non-Hodgkin’s lymphomas (NHL) including MCL and double hit lymphoma (DHL). Lantern’s LP-284 program has been accelerated and de-risked using A.I. insights and biological modeling powered by RADR®. Lantern has been able to advance LP-284 from initial RADR® insights regarding anti-cancer activity and potential mechanisms of action in hematological cancers, to selection of specific subtypes of lymphomas with superior response, to late stage IND enabling studies and initial design of first in human clinical trials in less than 2 years.
About Lantern Pharma:
Lantern Pharma (NASDAQ: LTRN) is a clinical-stage oncology-focused biopharmaceutical company leveraging its proprietary RADR® A.I. and machine learning platform to discover biomarker signatures that identify patients most likely to respond to its pipeline of genomically-targeted therapeutics. Lantern is currently developing four drug candidates and an ADC program across twelve disclosed tumor targets, including two phase 2 programs. By targeting drugs to patients whose genomic profile identifies them as having the highest probability of benefiting from the drug, Lantern's approach represents the potential to deliver best-in-class outcomes.
Forward-looking Statements:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, among other things, statements relating to: future events or our future financial performance; the potential advantages of our RADR® platform in identifying drug candidates and patient populations that are likely to respond to a drug candidate; our strategic plans to advance the development of our drug candidates and antibody drug conjugate (ADC) development program; estimates regarding the development timing for our drug candidates and ADC development program; expectations and estimates regarding clinical trial timing and patient enrollment; our research and development efforts of our internal drug discovery programs and the utilization of our RADR® platform to streamline the drug development process; our intention to leverage artificial intelligence, machine learning and genomic data to streamline and transform the pace, risk and cost of oncology drug discovery and development and to identify patient populations that would likely respond to a drug candidate; estimates regarding patient populations, potential markets and potential market sizes; sales estimates for our drug candidates and our plans to discover and develop drug candidates and to maximize their commercial potential by advancing such drug candidates ourselves or in collaboration with others. Any statements that are not statements of historical fact (including, without limitation, statements that use words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "model," "objective," "aim," "upcoming," "should," "will," "would," or the negative of these words or other similar expressions) should be considered forward-looking statements. There are a number of important factors that could cause our actual results to differ materially from those indicated by the forward-looking statements, such as (i) the impact of the COVID-19 pandemic, (ii) the risk that our research and the research of our collaborators may not be successful, (iii) the risk that none of our product candidates has received FDA marketing approval, and we may not be able to successfully initiate, conduct, or conclude clinical testing for or obtain marketing approval for our product candidates, (iv) the risk that no drug product based on our proprietary RADR® A.I. platform has received FDA marketing approval or otherwise been incorporated into a commercial product, and (v) those other factors set forth in the Risk Factors section in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on March 10, 2022. You may access our Annual Report on Form 10-K for the year ended December 31, 2021 under the investor SEC filings tab of our website at www.lanternpharma.com or on the SEC's website at www.sec.gov. Given these risks and uncertainties, we can give no assurances that our forward-looking statements will prove to be accurate, or that any other results or events projected or contemplated by our forward-looking statements will in fact occur, and we caution investors not to place undue reliance on these statements. All forward-looking statements in this press release represent our judgment as of the date hereof, and, except as otherwise required by law, we disclaim any obligation to update any forward-looking statements to conform the statement to actual results or changes in our expectations.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221215005355/en/
Contacts
Nicole Leber
Investor Relations Associate
ir@lanternpharma.com
Please find more information at:
Website: www.lanternpharma.com
LinkedIn: https://www.linkedin.com/company/lanternpharma/
Twitter: @lanternpharma
Monthly Newsletter: Sign-up here












