Independent dermatology research experts at Monasterium Laboratory GmbH tested, analyzed and validated various injection parameters using donated human tissue
VANCOUVER, BC / ACCESSWIRE / July 26, 2022 / RepliCel Life Sciences Inc. (OTC PINK:REPCF)(TSXV:RP)(FRA:P6P2) ("RepliCel" or the "Company"), a company developing next-generation technologies in aesthetics and orthopedics, announced today it has now received and analyzed all data and images from independent laboratory testing of the DermaPreciseTM injector prototype and consumables evaluating the injection systems' various depths and doses in donated human skin tissue.
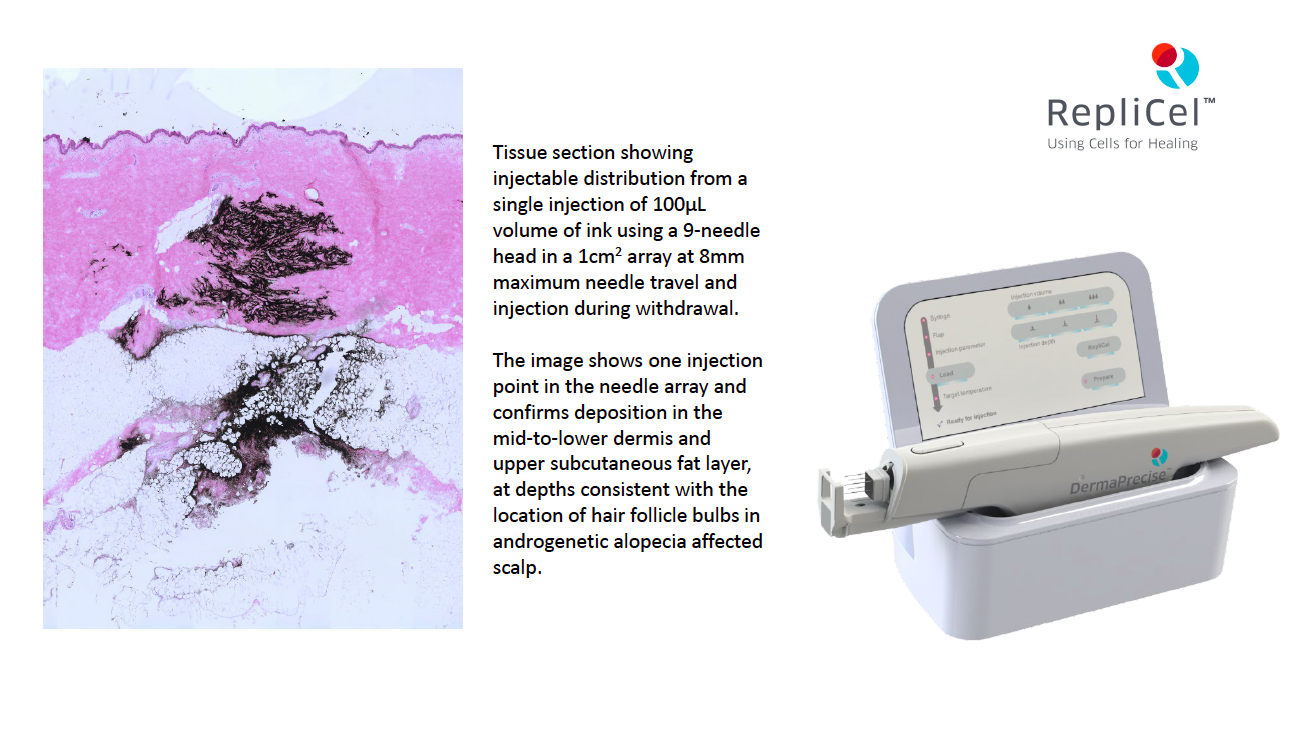
Monasterium Laboratory, a German-based laboratory specializing in dermatology research, tested intradermal and subcutaneous injections of different volumes to provide evidence of whether or not DermaPrecise was capable of delivering controlled and consistent injections as observed, recorded, and measured in donated, human skin tissue.
Human full thickness skin biopsies were injected with marker ink with viscosity matching commonly used drugs. Post-injection, the skin was cut into sections to be stained and evaluated. The injection parameters for depth and volume showed test results meet or surpass DermaPrecise objectives for consistency of volume and depth delivery. Sample pictures accompanying this press release show the kind of imagery captured in data accumulation pertaining to the injections.
"Analysis of the tests performed, confirms the unique injection consistency of the DermaPrecise™ injection system within a specified range in the tissue. With manual injections using a standard needle and syringe there is no guaranteed consistency in terms of injection depth or volume, but with the DermaPrecise™ there is uniform injection which allows for a large area of skin to be injected with the same amount of active substance in every single injection," stated Kevin McElwee, RepliCel's Chief Science Officer.
"What we are starting to see with these tests on donated human skin is evidence that the system is performing in accordance with the design, engineered functionality and intended uses of the DermaPreciseTM system," stated R. Lee Buckler, President and CEO of RepliCel Life Sciences, which developed and owns all rights to the DermaPreciseTM product portfolio and its underlying technologies. "It is intended to deliver a high level of control and consistent precision for intra-dermal and subcutaneous injections," he continued.
"As we continue to prepare for commercial launch of the DermaPreciseTM product line, it is critical we have these kinds of independent validation of the injection parameters and our ability to deliver best-in-class precision to our end users. Testing with donated human skin samples is a valuable tool in convincing clinicians to evaluate new technologies in real clinical treatment settings."
Dermatologist and clinical researcher Dr. Rolf Hoffmann, who has been a clinical advisor for the DermaPrecise™ development, stated: "As clinicians learn more about the mechanisms and effects of the various types of substances we inject into the dermis and subcutaneous tissue - including neurotoxins, fillers, antibody therapeutics, fat-dissolving enzymes, cell therapies, and other injectables - they are increasingly realizing that precision of depth, dose and/or delivery matters more to efficacy than was often originally believed."
About Monasterium Laboratory GmbH
Monasterium Laboratory GmbH, located in Münster Germany, is a fast-growing, dermatological research company specialized in preclinical skin, hair, pigmentation, neuroendocrinology, immunology, and wound healing research in the human system.
* Note: All experiments on human tissue are performed according to Helsinki guidelines and samples are collected after patient consent and ethics approval under relevant jurisdiction laws.
About the DermaPreciseTM Injector Product Line
The DermaPreciseTM Injector platform is an electronic injection system which will bring new levels of control over intra-dermal or subcutaneous injections where precision of depth, dose and/or delivery consistency matters.
About RepliCel Life Sciences
RepliCel is a regenerative medicine company focused on developing cell therapies for aesthetic and orthopedic conditions affecting what the Company believes is approximately one in three people in industrialized nations, including aging/sun-damaged skin, pattern baldness, and chronic tendon degeneration. These conditions, often associated with aging, are caused by a deficit of healthy cells required for normal tissue healing and function. These cell therapy product candidates are based on RepliCel's innovative technology, utilizing cell populations isolated from a patient's healthy hair follicles
The Company's cell therapy product pipeline is comprised of RCT-01 for tendon repair, RCS-01 for skin rejuvenation, and RCH-01 for hair restoration. RCH-01 has been the subject of successful safety and dose-finding clinical studies and is now the subject of its third clinical study evaluating efficacy for the treatment of male and female hair loss due to androgenetic alopecia. This ongoing study is being funded by Shiseido Company Limited pursuant to a license agreement which has now been terminated, but is the subject of an arbitration regarding Shiseido's rights to the product for Asia. RepliCel maintains the undisputed rights to RCH-01 for the rest of the world. RCT-01 and RCS-01 are exclusively licensed in Greater China to YOFOTO (China) Health Company. RepliCel and YOFOTO are currently co-developing these products in China. RepliCel maintains the rights to these products outside of Greater China.
RepliCel has also developed a proprietary injection device (DermaPreciseTM) and related consumables, which is expected to improve the administration of its cell therapy products and certain other injectables. YOFOTO has exclusively licensed the commercial rights for the DermaPrecise™ device and consumables in Greater China for dermatology applications and is expected to first launch the product in Hong Kong upon it being approved for market launch in either the United States or Europe. Please visit replicel.com for additional information.
Notable Facts:
- RepliCel's three cell therapy products have now been tested in over 100 patients in four countries on three continents.
- RepliCel now has key strategic regional partners each of which are now investing heavily in the further clinical testing and development of RepliCel's products for their markets. Data from each of the clinical programs will strengthen the product development initiatives for RepliCel and its other partners worldwide.
For more information, please contact:
Lee Buckler, CEO and President
604-248-8693
info@replicel.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
SOURCE: RepliCel Life Sciences, Inc.
View source version on accesswire.com:
https://www.accesswire.com/709732/Independent-Laboratory-Test-Results-Confirm-Value-of-DermaPrecise-TM-Injection-Consistency-in-Clinical-Simulation-Testing













