HOPE Therapeutic Subsidiary Focused on Developing a Best-in-Class Network of Clinics for Patients with Suicidal Depression and Related Disorders. HOPE is Planned to be Spun Out as a Separate Company to be Owned by NRXP Shareholders, and New Investors. Effort to be Funded Apart from NRXP.
WILMINGTON, Del. - Aug. 15, 2024 - PRLog -- For more information on $NRXP visit: https://www.nrxpharma.com/ OR https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
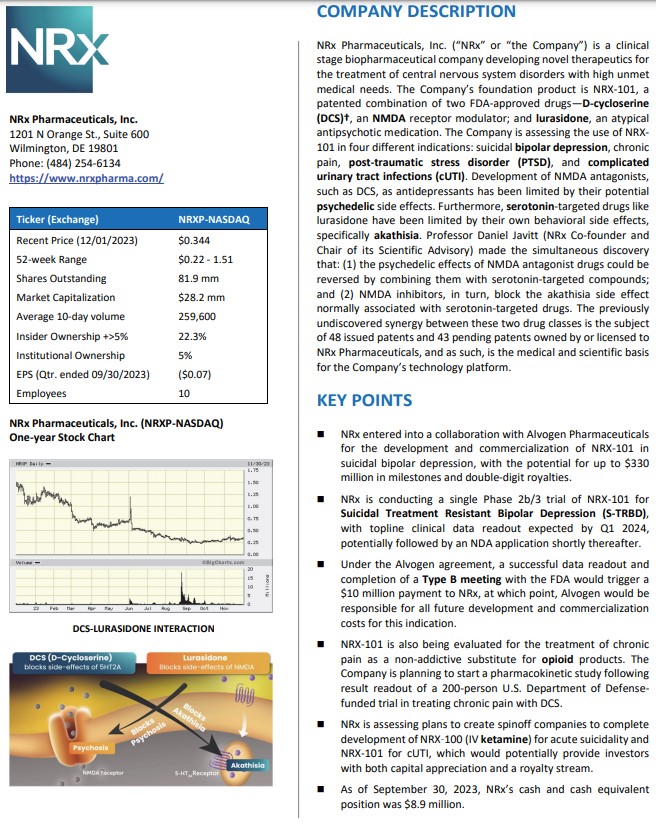
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain.
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
HIGHLIGHTS
Developing Therapeutics for the Treatment of CNS Disorders, Specifically Suicidal Bipolar Depression, Chronic Pain and PTSD.
Company Funded For New Drug Applications (NDAs) for NRX-100 (ketamine) and NRX-101
Audit of HOPE Therapeutics Subsidiary Complete, SEC Filing of Spinout Planned for Current Quarter.
Secured $10.8 - $16.3 Million Convertible-Debt Funding from an Institutional Investor.
NRX-100 NDA for Suicidal Depression Based on Data from Four Clinical Trials in Nearly 1000 Participants Demonstrating Highly Significant Efficacy.
Ketamine Findings Confirmed in Published 43,000 Person Cohort Study.
Phase 2b/3 Trial Data Presented at the American Society of Clinical Psychopharmacology. Profile Demonstrates Possible Best in Class.
Plans to file New Drug Application for Accelerated Approval under Breakthrough Therapy Designation and Priority Review of NRX-101.
Stability Data Continues to Mature on Three Manufacturing Lots Required for the NRX-100 (IV ketamine) NDA filing.
Company Announced Alignment with FDA on its Pediatric Study Plan for NRX-100, Also a Requirement for Filing of NDA.
Media Contact
Company Name: NRx Pharmaceuticals, Inc.
Contact Person: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Country: United States
Website: https://www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Photos: (Click photo to enlarge)




Source: Corporate Ads
Read Full Story - Substantial Funding, Debt Resolution, HOPE Dividend, Successful Clinical Trial Towards Accelerated Drug Approval for Bipolar Depression: Nasdaq: NRXP | More news from this source
Press release distribution by PRLog












