
Iovance Biotherapeutics Inc. (NASDAQ: IOVA) shares spiked over 20% on its advanced-stage melanoma treatment application to the U.S. Food and Drug Administration (FDA). The company submitted, and the FDA accepted, its Biologics License Application (BLA) for advanced melanoma. Iovance is a clinical-stage biotechnology company that develops novel immunotherapy treatments for various cancers. Immunotherapy is a form of personalized medicine. There are many types of immunotherapy, including monoclonal antibodies, immune checkpoint inhibitors and T-cell transfer, which is what Iovance uses.
T-Cell Transfer Immunotherapy
The T-cell transfer involves employing a patient's T-cells to help fight the cancer. This is also called adoptive cell therapy (ACT). Rather than focusing on trying to kill cancer cells like radiation and chemotherapy does, immunotherapy stimulates one’s natural immune responses to find and expunge cancer cells. Immunotherapy is better tolerated and more targeted than radiation and chemotherapy, which nukes all cells indiscriminately, healthy or cancerous.
What is Melanoma
Melanoma is a form of skin cancer that starts from melanocyte cells. These cells produce melanin which gives color to eyes, hair and skin. It can spread quickly and metastasize throughout the body if not detected early. When it spreads to the organs or lymph nodes, it's called metastatic melanoma, also known as stage IV melanoma. There are over 99,000 new cases of melanoma annually and over 7,600 deaths in the U.S.
Current Melanoma Treatments
Surgery is the most common treatment if detected early as they attempt to remove the tumor. Chemotherapy can be used to kill cancer cells or slow their growth. Radiation treatment attempts to kill cancer cells with high-energy beams and is often used when its spreads to the brain, body and other body parts. Immunotherapy is another type of treatment. While immunotherapy has effectively treated blood cancers, there's still much contention regarding its effectiveness with solid tumors.
Lifileucel
Lifileucel is a personalized immunotherapy comprised of a patient's tumor-infiltrating lymphocytes (TILs), a type of white blood cell believed to be critical in the immune response to cancer. TILs have receptors that recognize and bind to molecules in the cancer cells releasing proteins to kill them. Lifileucel is developed by extracting a patient's TILs, expanding them in the lab to make them special white blood cells and reinjecting them back into the patient. It helps the body to detect and attack cancer cells.
Market Potential
The total addressable market for Lifileucel is $1.2 billion, as the increasing number of metastatic melanoma patients is driving demand for immunotherapy treatments. The global melanoma market is expected to grow to $9.5 billion by 2030. Lifileucel can be combined with Keytruda as a first-line or a last-line of defense. Most significant is that the potential market for treating solid tumors is vast. Iovance estimates that 90% of all cancer cases are solid tumors, and 1.7 million new solid tumors occur annually in the U.S.
What’s Next?
Iovance has around $471 million in cash, which should be sufficient to cover its cash burn for the next 12 months. It has an action date of Nov. 25, 2023. It was previously granted a priority review and incidentally doesn't plan on an advisory committee meeting. The FDA decision's targeted date of action is November 25, 2023. It will be the first TIL approved for treatment in solid tumors if approved. The company is investigating other applications like non-small cell lung cancer and cervical cancer.
Takeover?
There’s always the possibility of a buyout from a larger pharmaceutical company already invested in melanoma treatments. Merck & Co. Inc. (NYSE: MRK) has been doing paired treatments of its Keytruda with Iovance and Moderna Inc. (NASDAQ: MRNA). Bristol Myers-Squibb Co. (NYSE: BMY) has Opdivo, an FDA-approved treatment for progressive metastatic melanoma by blocking the PD-1 protein on t-cells. Pfizer Inc (NYSE: PFE) and Novartis AG (NYSE: NVS) also have FDA-approved targeted therapy and immunotherapy treatments for melanoma. A takeover possibility would likely be contingent on full FDA approval.
Iovance Therapeutics analyst ratings and price targets can be found on MarketBeat.
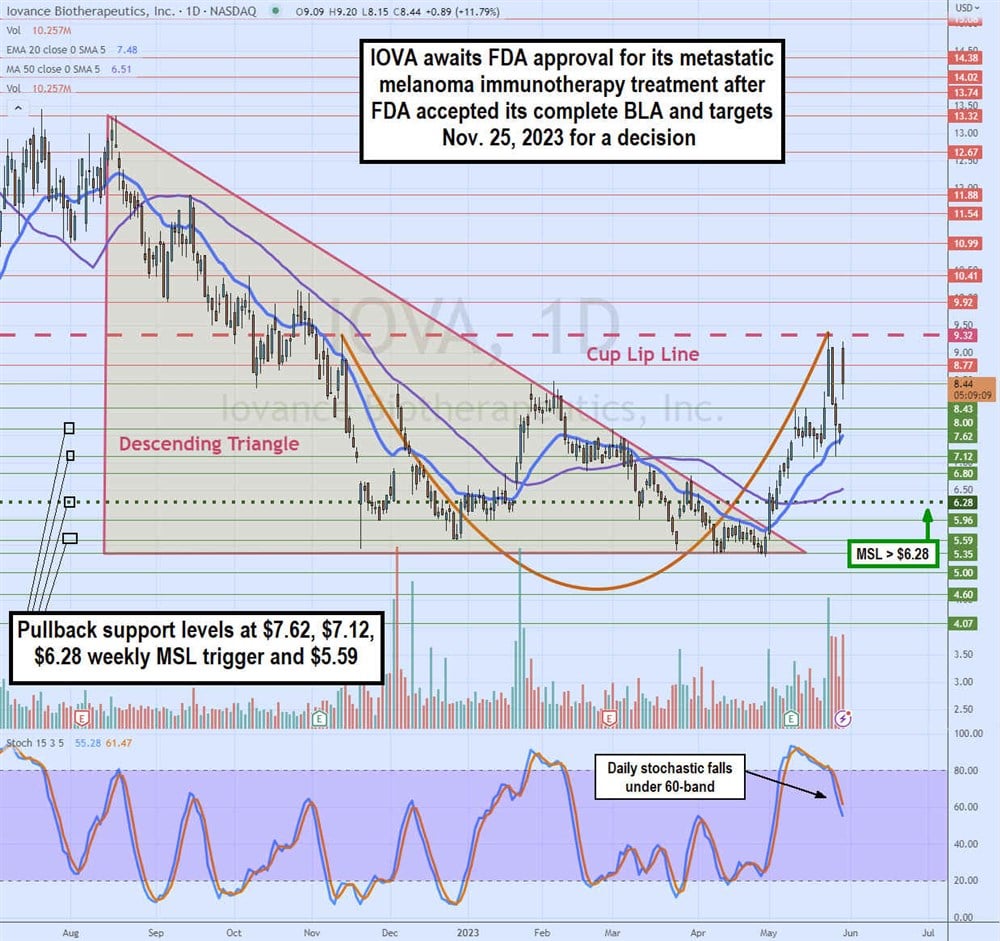
Daily Ascending Triangle Breakout and Cup Formation
The daily candlestick chart for IOVA was in a descending triangle starting at $13.32 on Aug. 15, 2022, as it fell to a low range flat-bottom around $5.35 in April 2023. IOVA also started forming the cup lip line after peaking at $9.32 on November 11, 2022. IOVA staged a rally on the breakout through its daily market structure low (MSL) buy trigger of $6.28 on May 2, 2023. Shares rallied to retest the daily cup lip line at $9.32 before pulling back to form a handle at $7.12. The daily stochastic continues to fall through the 60-band. The daily 20-period exponential moving average (EMA) support sits at $7.47, followed by the 50-period MA at $6.51. Pullback supports are at $7.62, $7.12, $6.28 daily MSL trigger and $5.59.













