eGenesis, a biotechnology company developing human-compatible engineered organs to address the global organ shortage, today announced the close of a $191 million Series D financing. Proceeds will be used to advance the company’s lead product candidate, EGEN-2784, to a first-in-human study for kidney transplant. The funding will also be used to advance pipeline programs as well as scale production.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240904466099/en/
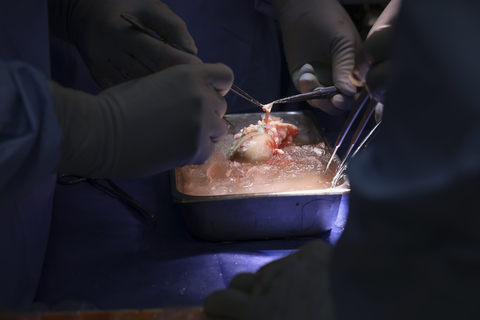
Surgeons prepare the eGenesis porcine kidney for transplantation. (Photo credit: Massachusetts General Hospital)
The financing was led by Lux Capital, with participation from existing investors ARCH Ventures, Khosla Ventures, Farallon Capital Management, Alta Partners, Fresenius Medical Care Ventures, and Leaps by Bayer as well as new investors DaVita, Eisai Innovation, NATCO Pharmaceuticals, and Parkwood Corporation.
“This financing demonstrates the confidence that investors have in cross-species transplantation as a potential solution to the global organ crisis and in eGenesis as the leader in this sector,” said Mike Curtis, President & CEO, eGenesis. “We are thrilled to work with Lux Capital and this world-class syndicate to advance our lead program to formal clinical trials. With this support, we are one step closer to bringing a solution to help patients suffering from organ failure.”
In March 2024, eGenesis announced the world’s first successful porcine kidney transplant in a living patient. The transplant was authorized by the U.S. Food & Drug Administration (FDA) under the Expanded Access pathway and performed by a surgical team at Massachusetts General Hospital.
“Decades of progress in cross-species transplantation, accelerated by the evolution of modern genome editing tools and next-generation sequencing, have enabled eGenesis to advance genetically engineered organs to the clinical setting,” said Peter Hebert, Co-Founder and Managing Partner, Lux Capital. “What was once science fiction is now science fact. We are thrilled to support eGenesis in advancing this pivotal modality to address one of the most intractable challenges facing medicine today – the global organ shortage crisis.”
More than 800,000 people in the U.S., and millions globally, suffer from end-stage renal disease or kidney failure, a life-threatening condition for which transplantation is considered the gold standard treatment option to improve quality of life and outcomes. Yet the demand for organs far outpaces supply, with more than 90,000 individuals on the kidney waitlist and only approximately 25,000 kidney transplants performed each year.
Human compatible donor organs developed by eGenesis offer a potentially viable alternative to end waitlist mortality and alleviate the shortage of transplantable organs.
The eGenesis donor kidney (EGEN-2784) is the company’s lead candidate for kidney transplant and carries three classes of edits: (1) knock out of three genes involved in the synthesis of glycan antigens implicated in hyperacute rejection, (2) insertion of seven human transgenes involved in the regulation of pathways that modulate rejection: inflammation, innate immunity, coagulation, and complement, and (3) inactivation of the endogenous retroviruses in the porcine genome.
eGenesis is the only company in the industry developing organs that carry all three classes of edits to address organ safety and efficacy. Without genetic modification, a porcine kidney would be immediately rejected by a human recipient.
About eGenesis
eGenesis is pioneering a genome engineering-based approach in the development of safe and effective transplantable organs to end the global organ shortage and transform the treatment of organ failure. The eGenesis Genome Engineering and Production (EGEN™) Platform is the only technology of its kind to comprehensively address cross-species molecular incompatibilities and viral risk via genetic engineering to improve the lives of patients in need of a transplant. eGenesis has demonstrated durable preclinical success to date and is advancing development programs for kidney transplant, acute liver failure, and heart transplant. Learn more at www.egenesisbio.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240904466099/en/
Contacts
Media
Kimberly Ha
eGenesis
kimberly.ha@egenesisbio.com












