This Clinical Trial will Show the Safety and Probable Benefit of TETRANITE® for Replacing the Use of Metal Plates and Screws
RevBio, Inc., announced that it has received approval from the U.S. Food and Drug Administration to start a 20-patient pilot clinical trial to examine the safety and efficacy of the company’s bone adhesive biomaterial called TETRANITE® to immediately stabilize and fixate cranial flaps following craniotomy procedures associated with brain surgery. This approval was largely predicated on the successful pivotal preclinical and surgeon handling testing conducted by the company.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231208749209/en/
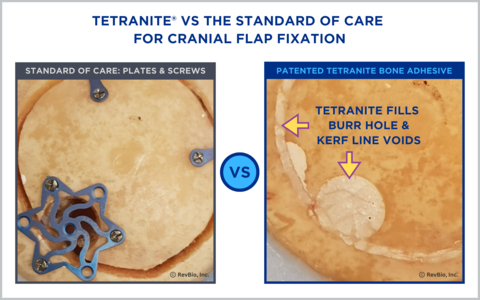
On the left is an image of standard metal fixation hardware consisting of bur hole covers and plates and screw systems that are used to re-affix cranial flaps following craniotomy procedures. On the right is TETRANITE® which fills the missing bone, seals the site from infection, and over time is resorbed and replaced with bone as demonstrated in RevBio’s pre-clinical testing. (Graphic: Business Wire)
The clinical trial will be conducted by Timothy R. Smith, MD, PhD, MPH, Director, Computational Neuroscience Outcomes Center and practicing neurosurgeon at Brigham and Women’s Hospital in Boston, Massachusetts, and L. Madison Michael, MD, Co-Director of Cranial Base Surgery for the Methodist Brain and Spine Institute and practicing neurosurgeon at the Semmes Murphey Clinic in Memphis, Tennessee.
“We are looking forward to evaluating the efficacy of the product that could provide immediate, rigid fixation that would be preferable to existing plates and screws, which are the current standard of care,” said Dr. Smith. “We’re excited at the potential that this product could provide a biological adherence between the bone flap edges and the skull, as well as an architecture for osteogenesis over time and look forward to the results of the clinical trial.”
Craniotomy procedures are required to remove a brain tumor, treat an aneurysm, relieve intracranial pressure, or perform deep brain stimulation. The instruments used to cut into the skull leave a kerf line, which is a continuous gap between the bone flap, which is temporarily removed to conduct the brain surgery, and the surrounding skull bone. As part of the surgical closure process, the cranial flap is secured back into place with plates and screws. The kerf line, however, is typically not sealed, which compromises the ability of the flap to integrate with the surrounding bone. The open kerf line also leaves a channel for leakage of cerebrospinal fluid (“CSF”) that can lead to devastating infections. Infection rates in published literature for certain regions of the skull range up to 21%, which most often results in meningitis, subdural empyema, or cerebral abscesses.1,2 CSF leaks increase the risk of infection by as much as 13 times and are considered the most significant risk factor for post-operative meningitis.3 CSF leakage has been reported to almost double the healthcare cost of the original procedure.4 Furthermore, the use of hardware fixation devices does not provide a scaffold for biological bone healing and also often leads to readmission and secondary surgeries to correct issues associated with pain and protrusion of the metal hardware. The use of TETRANITE could offer an innovative alternative approach for cranial flap fixation that eliminates the use of hardware fixation products and provides a more natural solution that improves bone healing, enhances the conformal contour of the skull, and, at the same time, provides a sealant to resist CSF leaks that frequently lead to infection.
“We are truly excited to move this project into the clinical phase of testing,” said Brian Hess, CEO and Founder of RevBio. “We envision that the use of TETRANITE will become the standard of care given all the benefits this biomaterial can provide in comparison to traditional plates and screw systems.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called TETRANITE®. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
_____________________________
1 Dashti, S. R.; Baharvahdat, H.; Spetzler, R. F.; Sauvageau, E.; Chang, S. W.; Stiefel, M. F.; Park, M. S.; Bambakidis, N. C. Operative Intracranial Infection Following Craniotomy. Neurosurg. Focus 2008, 24 (6), E10. https://doi.org/10.3171/FOC/2008/24/6/E10.
2 Mollman, H. D.; Haines, S. J. Risk Factors for Postoperative Neurosurgical Wound Infection. A Case-Control Study. J. Neurosurg. 1986, 64 (6), 902–906. https://doi.org/10.3171/jns.1986.64.6.0902.
3 Ibid.
4 Piek, J.; Weber, C.; Kundt, G.; Tronnier, V.; Spuck, S.; Hirdes, C.; Kehler, U.; Ditges, C. Pharmacoeconomical Consequences of Postoperative CSF Leaks after Intracranial Surgery--a Prospective Analysis. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2012, 73 (1), 25–28. https://doi.org/10.1055/s-0032-1304501.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231208749209/en/
“We are looking forward to evaluating the efficacy of the product that could provide immediate, rigid fixation that would be preferable to existing plates and screws, which are the current standard of care,” said Dr. Smith.
Contacts
Michael Tiedemann
mtiedemann@revbio.com












