− NorthStar is further expanding commercial-scale domestic radioisotope production capabilities using IBA’s environmentally preferable Rhodotron® accelerator technology –
NorthStar Medical Radioisotopes, LLC (‘NorthStar’), a global innovator in the development, production and commercialization of radiopharmaceuticals used for therapeutic applications and medical imaging, and IBA (Ion Beam Applications S.A., EURONEXT), the world leader in particle accelerator technology, today announced a new agreement under which NorthStar will purchase two additional Rhodotron® TT300 HE electron beam accelerators, and the associated beamlines, from IBA for the production of molybdenum-99 (Mo-99).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220920005229/en/
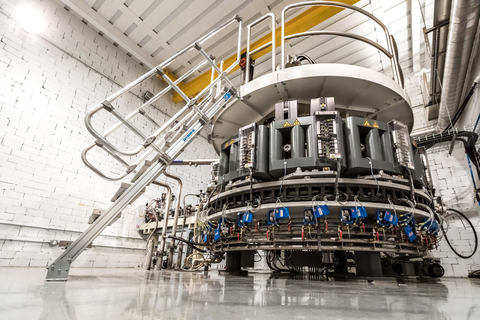
IBA Rhodotron®TT300-HE electron beam accelerator (Photo: Business Wire)
The purchase marks a total of five Rhodotron® accelerators that NorthStar has purchased from IBA to date. NorthStar previously purchased two electron beam accelerators from IBA in 2019 for the production of Mo-99, and purchased a third accelerator in 2021 for production of the therapeutic radioisotope actinium-225 (Ac-225). The additional accelerators will be used to further expand NorthStar’s commercial-scale radioisotope production capabilities at its Beloit, Wisconsin headquarters. All NorthStar production processes employ innovative, environmentally preferable technology and are non-uranium based.
NorthStar’s first Accelerator Production facility expansion in Beloit is nearing completion and moving to the final phase of activities required for licensure and FDA approval. Both accelerators are operating at full power and undergoing final testing. Equipment in the adjacent Isotope Processing facility has been installed and is undergoing final testing. Construction of NorthStar’s dedicated Actinium-225 Production facility is also well underway, with shipment of the third IBA Rhodotron® accelerator expected by the end of 2022.
“NorthStar continues to invest in the future of nuclear medicine using innovative technology to advance environmentally sustainable radioisotope production, and the purchase of these additional accelerators marks another milestone of our highly productive relationship with IBA,” said Stephen Merrick, Chief Executive Officer of NorthStar. “IBA has demonstrated extensive commercial expertise and excellent performance in delivering electron beam accelerators for our Mo-99 production expansion program, and in the design and custom-build of our Ac-225 accelerator. We are proud that IBA is a partner in helping to make these important diagnostic imaging and therapeutic radioisotope products available to advance patient health, and look forward to continuing to work with them.”
Olivier Legrain, Chief Executive Officer of IBA commented, “We are delighted to sign this latest agreement with NorthStar Medical Radioisotopes and to continue to deliver innovative solutions for reliable radioisotope supply. IBA’s Rhodotron® accelerators provide the most advanced electron accelerator technology in the world, enabling a non-uranium based and highly efficient method for producing medical radioisotopes such as Mo-99, Ac-225 and Cu-67. We look forward to continuing to work with NorthStar to advance research and help patients.”
About IBA
IBA (Ion Beam Applications S.A.) is the world leader in particle accelerator technology. The company is the leading supplier of equipment and services in the field of proton therapy, considered to be the most advanced form of radiation therapy available today. IBA is also a leading player in the fields of industrial sterilization, radiopharmaceuticals and dosimetry. The company, based in Louvain-la-Neuve, Belgium, employs approximately 1,600 people worldwide. IBA is a certified B Corporation (B Corp) meeting the highest standards of verified social and environmental performance.
IBA is listed on the pan-European stock exchange EURONEXT (IBA: Reuters IBAB.BR and Bloomberg IBAB.BB).
More information can be found at: www.iba-worldwide.com.
About NorthStar Medical Radioisotopes, LLC (NorthStar)
NorthStar Medical Radioisotopes is a commercial-stage nuclear medicine company that manufactures and distributes diagnostic and therapeutic radioisotopes and radiopharmaceuticals. The Company’s proprietary state-of-the-art technology and proven management team have propelled it to the forefront of U.S. medical radioisotope production as the sole domestic producer of the diagnostic imaging radioisotope molybdenum-99 (Mo-99). Mo-99 is used to generate technetium-99m (Tc-99m), the standard of care in diagnostic imaging to assess the extent and severity of heart disease and cancer. NorthStar’s unique Mo-99 production process is non-uranium based and environmentally friendly. NorthStar is expanding its industry-leading position in the emerging area of therapeutic radioisotopes, which are used in targeted radiopharmaceutical therapy to treat cancer, respiratory and other diseases. Using first-in-kind and environmentally-sound electron accelerator technology, NorthStar is poised to be the first commercial-scale producer of therapeutic radioisotopes actinium-225 (Ac-225) and copper-67 (Cu-67). NorthStar also collaborates with other companies in the development of radiopharmaceuticals. For more information about NorthStar’s comprehensive radiopharmaceutical portfolio, visit: www.northstarnm.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220920005229/en/
Contacts
For NorthStar Medical Radioisotopes, LLC
Corporate:
Lisa Holst
Vice President Sales and Marketing
678-471-9027
lholst@northstarnm.com
Investor Relations:
Paul Estrem
Executive Vice President and Chief Financial Officer
608-987-8318
pestrem@northstarnm.com
Media:
Priscilla Harlan
781-799-7917
pharlan@shiningrockllc.com
For IBA
Soumya Chandramouli
Chief Financial Officer
+32 10 475 890
Investorrelations@iba-group.com
Olivier Lechien
Corporate Communication Director
+32 10 475 890
communication@iba-group.com
Consilium Strategic Communications
Amber Fennell, Angela Gray, Lucy Featherstone
+44 (0) 20 3709 5700
IBA@consilium-comms.com












