The designation is a result of UNEEG's strong focus on developing novel disease management support tools with transformational and first-to-market potential
To qualify for Breakthrough Device Designation from the US Food and Drug Administration (FDA), a device technology must address an unmet need and provide evidence that it has the potential to provide for more effective treatment of patients - or as stated, "substantial clinical improvement".
FDA Breakthrough Device Designation is granted to UNEEG's Subcutaneous EEG Implant System and will expedite the review of this innovative technology that can potentially improve lives of people with severe and even life-threatening epilepsy where medical treatment has not yet led to optimal outcomes.
As part of the Centers for Medicare & Medicaid Services stated commitments to ensuring beneficiary access to medical advancements with the potential to improve health outcomes and equity, upon FDA Clearance, the Breakthrough Designation may enable important temporary coverage and payment for qualifying novel medical technologies immediately following launch.
In addition to the US Breakthrough Designation, UNEEG' implantable device and second-generation wireless Bluetooth-assisted and Red Dot Award-winning device has just received European CE mark, after satisfying the European authorities' stringent Medical Device Regulation (MDR), now ready for launch.
COPENHAGEN, DENMARK / ACCESSWIRE / November 25, 2024 / ‘This designation will expedite our cooperation with FDA and potentially allow for faster FDA Marketing Authorization,' says Torben Sandgren, CEO of UNEEG medical. ‘Breakthrough Designation also opens up the possibility of immediately engaging with regulators and payers - making it possible for Americans with epilepsy to gain faster access to this groundbreaking technology.'

A person with uncontrolled epilepsy, fitted with the subcutaneous EEG implant and recorder in the recently launched European version.
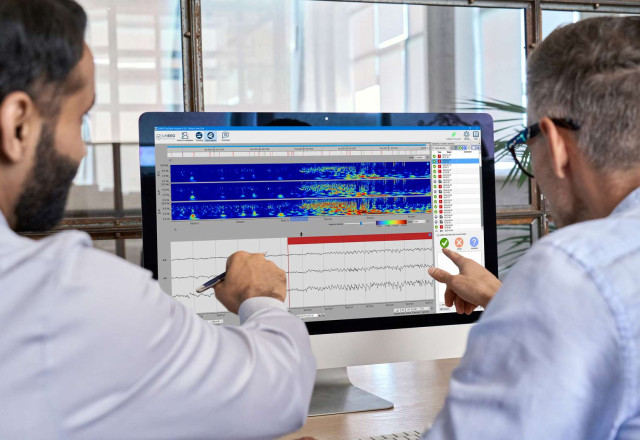
The Subcutaneous EEG Implant System supports physicians' epilepsy management by providing 24/7 brain waves recordings (EEG) via a small subcutaneous device behind the ear. The solution can record brain activity for months and year at a time.
The recorded EEG data is sent via a dedicated cloud solution to hospitals where proprietary AI-developed software identifies potential seizure activity. Such data gives physicians the ultra long-term, objective and actionable insights needed to optimize disease management - all while people with epilepsy continue to live their everyday lives with full mobility.
About UNEEG medical
We break new ground in epilepsy. UNEEG medical is an entrepreneurial and ambitious company specializing in providing the epilepsy community with more accurate knowledge of seizures to support improvement in epilepsy care. Our goal is to improve the quality of life for people with epilepsy. The first generation of our unique subcutaneous EEG solution for remote monitoring of brain activity was CE marked in 2019.
We are well underway with an FDA approved IDE-study (Investigational Device Exempt) at several leading universities in the US and Europe.
UNEEG medical employs approximately 70 people. The company is based in Denmark, with subsidiaries in Germany, the UK, Austria and the US.
Contacts:
Torben Sandgren, CEO, or Britta Johansen, Executive Assistant & Corporate Communication
Contact Information
Torben Sandgren
CEO
uneeg@uneeg.com
Britta Johansen
Executive Assistant & Corporate Communication
brjo@uneeg.com
Related Images
SOURCE: UNEEG medical