SEATTLE, WA / ACCESSWIRE / October 5, 2021 / EMulate Therapeutics, Inc. (EMulate) announced today the results of screening models targeting the central nervous system in animals and in first in human exposures. The first step in developing the company's proprietary ultra-low Radio Frequency Energy (ulRFE®) technology for multiple mental health medical indications. Signals are internally referred to as cognates by EMulate. These cognates were initially tested in mice at Porsolt SA in Le Genest-Saint-Isle, France, an independent Contract Research Organization (CRO) specializing in preclinical anxiety and depression models, as part of EMulate's scientific and business strategy to expand its ultra-low Radio Frequency Energy (ulRFE®) technology beyond the oncology and pain markets.
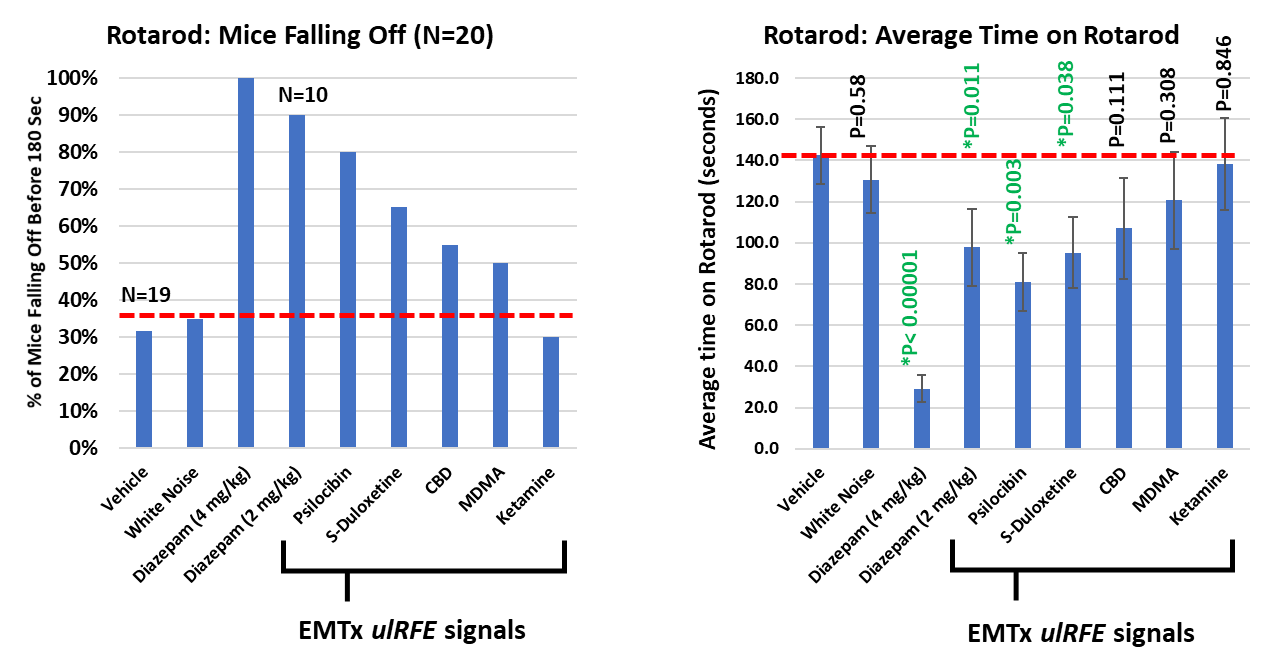
The Porsolt models assessed four broad areas: general behavior (Irwin Grid), locomotor activity (rotarod), elevated T-maze (anxiety) and forced swim test (depression). In the screen, eight cognate candidates with potential in reducing anxiety and/or depression were tested. Two of the eight cognates showed a significant effect (compared to vehicle) on locomotor activity, similar in effect to a low dose of diazepam (2 mg/kg). One cognate (CBD) was trending towards significance. The results also indicated a positive safety profile.
"As a therapist who treats patients with PTSD, anxiety, and depression, I have seen the value of psychoactive assisted therapy in greatly improving clinical outcomes. But today I need a psychiatrist in the room to administer certain drugs, such as ketamine, and I don't know what regulatory restrictions will be in place for MDMA and psilocybin in the future. My personal experience of the psychoactive derived cognates that I have tried is that these are similar experiences to the original psychoactive drugs and of great clinical potential. I look forward to participating in clinical trials to further document the mental health benefits this exciting new treatment option can offer," stated Manuela Mischke-Reeds, a San Francisco, CA-based psychotherapist.
"As our society faces growing mental health challenges, alternate treatments are being sought. These data, including results of initial human experiences, are extremely encouraging, exciting and a potential game changer for patients suffering from numerous mental health conditions," stated Chris Rivera, EMulate's President and CEO. "These data suggest that Radio Frequency Energy, focused on the ultra-low end of the energy spectrum (ulRFE), may be able to provide beneficial clinical effects in combination or potentially without the use of chemicals or drugs for many people suffering conditions including cluster migraines, severe post-traumatic stress disorder, depression and end-of-life anxiety."
"The ongoing expansion of our cognate pipeline and the diversification into the mental health space further demonstrates the flexibility of EMulate's technology. The preliminary results from Porsolt indicate that our cognates emulate the drugs and chemicals' desirable effects in mice without the drug or chemical being present. In addition, the safety profile of exposure on these cognates is acceptable for human testing," stated Dr. Xavier Figueroa, Vice President, Preclinical Development. Based on the results obtained from Porsolt, EMulate has identified cognate candidates to advance into more specific and sensitive testing models in preparation for human clinical trials potentially in 2022.
EMulate continues its efforts to expand the ulRFE® technology beyond the oncology market, and to develop the technology into a platform for applications in medical, veterinary, agricultural, non-medical and industrial applications.
About EMulate Therapeutics
EMulate Therapeutics is a clinical stage company utilizing proprietary ulRFE technology to provide safe and effective therapeutic benefits. The company has generated encouraging data in patients afflicted with glioblastoma and diffuse midline glioma. It has also generated encouraging preclinical data in pain and mental health models, as well as in animal health and bio-agriculture. EMulate Therapeutics is also the licensor of its proprietary technology to Hapbee Technologies, Inc. (HAPB:TSVX). Hapbee is a commercial stage consumer technology company.
Company Contact:
David Matteson
Cell: 425/478-2121
dmatteson@emultatetx.com
Investor Contact
James Carbonara
Hayden IR
Tel (646) 755-7412
james@haydenir.com
SOURCE: EMulate Therapeutics Inc
View source version on accesswire.com:
https://www.accesswire.com/666772/EMulate-Therapeutics-Announces-Positive-Pre-Clinical-Data-in-Mental-Health-Models-and-First-in-Human-Experience-Using-its-Proprietary-Radio-Frequency-Energy-Technology-Preliminary-Results-Emulate-Drug-Effects-in-Rodent-Models-and-in-Human-Exposures












